2015 blog cont’d…. Another massive campaign to discredit the authors of the PACE study into three ME/CFS interventions is underway. Over 12,000 signed the first petition to request retraction of supposed claims that some one quarter ‘recovered’ – even though the Lancet paper clearly states potential to just ‘moderately improve outcomes’, specified per the Mayo Clinical Significance Consensus. Another lobbying is underway, so far 6,300 have signed a demand to the GMC regulators that the study doctors be disciplined – suggesting 10 years custodial penalty for the crime of Fraud. The gist of concerns is than consequent to PACE report practitioners have distressed sufferers from ME/CFS with callous disregard. Claim is even made of “… harm done to children being forced to go to school and being subject to child protection plans“. This irrational outburst references the Tymes Trust’s Jane Colby regarding the potential for healthcare authorities to intervene so as to enforce adherence to clinical guidelines after PACE recommendations.
First in GP Sarah Myhill’s complaints beginning on pg 7 of 25 are that PACE “has effectively determined CFS/ME as a psychological condition“. Recall that the interventions detailed in the Lancet article were: Cognitive Based Therapy (CBT) with a psychologist; Graded Exercise Therapy (GET) with a physio; Adaptive Pacing (APT) with an Occupational Therapist (OT); and against a control group receiving standard care from a CFS specialist. CBT and GET were significantly better for fatigue and functioning, while APT was no better than the control/placebo. The authors state clearly: “The effectiveness of behavioural treatments does not imply that the condition is psychological in nature.” Dr Myhill’s 2012 reprimand by GMC and recently concluded cautionary period must be considered in grading her opinions, when the disciplinary ruling declared: “statements in relation to contraception and breast cancer screening that were factually incorrect; clinically unsubstantiated; and contrary to national guidelines. In so doing she used her position as a registered practitioner to exploit patients’ lack of medical knowledge by arousing ill found fears for their health.” Myhill’s website promotes powerlifting as High Intensity Training – but not for ME/CFS, where diet and detox are advised. And vaccinating is discouraged.
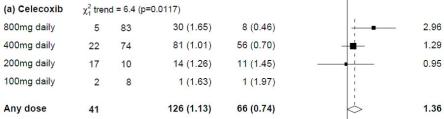
Another line of advocacy comes from Tuller, Geraghty, Wilshire et al. The link has a number of articles, including the PACE authors’ refuting of complaints re research quality. More telling is the activist’s collated manuscript ‘Rethinking the treatment of chronic fatigue syndrome’, which begins with an allegation that the Randomised Controlled Trial did not control nuisance variables, such as contact hours. This is alarming. ‘Control’ in this situation means that the intervention was compared with a control group, not their idea that control be applied so as to enforce participant compliance. The scientific complaints differ over time, but this paper zeroes in on statistical analysis in that the published protocol considers every possible comparison between therapies, and the Bonferroni principle requires stronger levels of proof ie the more permutations (ie 6 pairwise comparisons), the greater likelihood of a random fluke (odds of 1 in 125 actually) supporting the hypothesis of a therapy being better than the control ie standard care. The pairwise tests were a change to the stats plan, by dropping consideration of combo-therapy eg is APT and GET better than GET alone etc….explained as being overly convoluted.
Access to raw data was achieved by activist’s FoI request. Looking at their re-analysis of supplied data in Table1, people improved most under two therapies. No argument. The control group also did over time, where a placebo or Hawthorne effect can result from the satisfaction of working with supportive professionals towards a solution.
Expert opinions and agendas at play
Independent commentators are worth noting: OT Bronnie Thompson admires the study, but is concerned that their APT protocol failed to set goals to work towards. Their envelope of maximal activity was fixed within a ‘disabled identity’ focused on what COULDN’T be achieved, a problem noticed by Prof Leonard Jason. His Energy Envelope Theory relies upon success in avoiding crashes being inducement to better achievements in future. This is similar to Feldenkrais practice, progressing away from fear of movement through progressive challenges. Medical journalist Dr Norman Swan discusses the unprecedented outrage in the patient community with a study author and journal editor, but only considers the absence of harm during the trial (1% of all treatment arms reported worsening conditions). They suggest that activists hijacking the CFS community does them harm, without stopping to think about why there is even an outcry and whether their GPs are at fault.
It is obvious that distress results from unsympathetic doctors who’ve simplified the study conclusion as “get active, get counseling, and get out of my practice“. Indeed, practitioners proudly state conviction in their ability to discern CFS patient agendas. “I often use it as a diagnostic tool for MUPS (Medically Unexplained Physical Symptoms), that I get irritated by patients.” A vulnerable population then becomes prey to peddlers of solutions that are accompanied by rather more sympathetic caring. At a patient forum Dr Daniel Lewis agreed with Tuller’s complaint that the participant inclusion criteria of ‘Oxford Research’, rather than ‘Canadian Consensus’, was the problem. They weren’t suffering real ME/CFS, whatever that is. He sells meditation courses targeting chronic fatigue or pain in general however, without quibbling about specific diagnoses in attendees. Likewise a clinician’s summit unanimously supported their client’s grievances against PACE conclusions being given as guidance to doctors.
Personal injury specialist legal firm Maurice Blackburn sponsors Australia’s Emerge ME/CFS foundation, and seeks litigants who’ve been refused disability payment. They also advertise on SBS TV, who have requested patient’s stories for a program. CFS guru Dr Jacob Teitelbaum initially took a rational stance that PACE results were being misinterpreted in the media, but five years later joined the herd by stating in his blog: “… the PACE trial that wrongly concluded that CFS patients should be treated with psychotherapy.” Other experts such as Jose Montoya just focus on their research.
The study team took the controversy onboard, replying with rational argument to editorial letters. It then seemed that the time was ripe to shoot themselves in the foot. Perhaps the declared conflicted interest of team member’s consulting to insurance companies, presumably over disability payouts, made the Lancet article just a testing of the waters. Another writeup appeared, declaring ‘Recovery is possible!’ much as Chamberlain did in saying “Peace in our time”. And war broke out.
This wasn’t the only investigation into exercise as therapy for CFS. Last year’s update to the meta-analysis of 7 trials affirmed the results, every one of them showing benefit for reported Fatigue. But once again, researchers do themselves no favours with clinicians or patients: the publishing/editorial group is Cochrane’s ‘Common Mental Disorders’ .
My thoughts
CFS is an unmet challenge to medicine. There’s no fix, only symptom relief. LowDose Naltrexone relieves the brain fog (presumed to result from glial inflammatory response), beta-blockers may be used against POTS in orthostatic intolerance (light-headedness upon arising), and supplements such as CoQ10 or D-Ribose aid mitochondrial energy production. Post-exertional malaise is a constant however, which reinforces the lost sense of identity that was once based upon our function. Patient experience is of an invariant, and lifelong struggle. For anyone else we experience affliction as mostly transitory, even chronic illness can go into remission upon treatment. They who experience anxiety attacks, also know there’s moments of achievement. Joy counters sadness, emotions rise and fall again. It is possible to mentally step back and observe thoughts and sensations that may come and go, without attaching identity or sense of self to such temporary states. This is infinitely harder when the disease is so poorly understood.
CBT and GET offer slight improvement, a readiness for future solutions rather than idling whilst deconditioning – where practitioner’s pushing of anti-depressant meds worsens weight gain. Meanwhile however, you’re powerless prey to commercialism.
Right of Reply & Disclosure
Dr Lewis’ office has never replied to my correspondence. SBS passed a message on to their producer. David Tuller wrote back that the trial authors assumption of the CFS sufferer’s [de]reconditioning biased their choice of interventions offered, which is fair comment. If only there were better answers to this perplexing problem.
I’m married to a MUPS, and like others I know well, am struck by their hitherto overachieving.









 Cochrane pioneers Gotzsche and Chalmers are much alike in their evidence fudging. The
Cochrane pioneers Gotzsche and Chalmers are much alike in their evidence fudging. The